The EU Battery Regulation, which went into effect in August 2023, mandates the declaration of carbon footprints (CFP) and high levels of environmental consideration throughout the product life cycle, necessitating a swift response from companies across the globe. In response, Toshiba has developed a direct recycling method for lithium-ion battery oxide anodes at low cost and with low environmental impact through a simple heat treatment process.
The method leverages the characteristics of oxide active materials with stable crystal structures, and utilizes direct recycling, which recycles active materials as they are and without reverting them to chemical element. Active materials are substances that store and release electricity through redox reaction. The active material is coated on a thin metal sheet called a “current collecting foil.” This recycling method utilizes the stability of the active material structure and enables the active material to be separated from the current collecting foil of the negative electrode in that state by performing a simple heat treatment. Because the structure of the active material is stable, there is no need for complex reactivation processes, thereby enabling the reuse of active materials at low cost. Additionally, compared with conventional electrode recycling methods, the method can be carried out at lower temperatures, thereby reducing the environmental impact. Comparing materials recycled using this method to virgin materials that have never been used, Toshiba estimates up to an 85% reduction in the carbon footprint (CFP).
Furthermore, Toshiba applied this method to the niobium titanium oxide (NTO) anode batteries developed by the company. Performance evaluations of batteries made with recycled electrodes confirmed that the electrodes maintain more than 97% of capacity, comparable to new electrodes. The batteries also showed the same long life as new batteries.
Development background
As the world moves toward a carbon-neutral society, the electrification of automobiles and various other forms of mobility is progressing, making lithium-ion batteries an indispensable component.
Against this background, the EU Battery Regulation went into effect last year to promote resource circulation and reduce environmental impact. Similarly, Japan’s Ministry of Economy, Trade and Industry launched a study group aimed at developing a supply chain for batteries in 2022, reflecting a global increase in the demand for lithium-ion battery recycling and reduction of product CFPs.
The recycling of cathode materials containing cobalt and nickel has progressed in response to this demand, but the recycling of graphite, a common lithium-ion battery anode material, has not advanced due to the complexity of the processes required for recycling, which arise from structural changes and degradation after long-term use, presenting significant cost challenges. However, like cathodes, anodes have a definite CFP and there is a need to reduce this through recycling, giving rise to a demand for the development of a simpler recycling method. Toshiba has developed batteries with oxide anodes that are suitable for commercial vehicles expected to be electrified in the future, offering higher power and longer life than batteries with graphite anodes. Toshiba has developed a recycling method that takes advantage of the features of oxide anodes, which are easier to recycle compared with graphite.
In recycling the active materials of batteries, there are methods such as direct recycling, which reuse the active material while maintaining its structure, and methods that re-synthesize active materials after breaking them down into their elements and recovering them. Recycling that involves breaking the active material down to chemical elements require energy to resynthesize it for use, while direct recycling does not require resynthesis, thereby reducing both cost and environmental impact, which has spurred research and development in recent years.
Features of the technology
In light of these needs, Toshiba has developed a direct recycling technology for high-power, long-life oxide anode batteries. This technology has been verified to separate the active material from the current collecting foil of the oxide anode electrodes while maintaining the structure and properties of the active material, thereby enabling direct reuse.
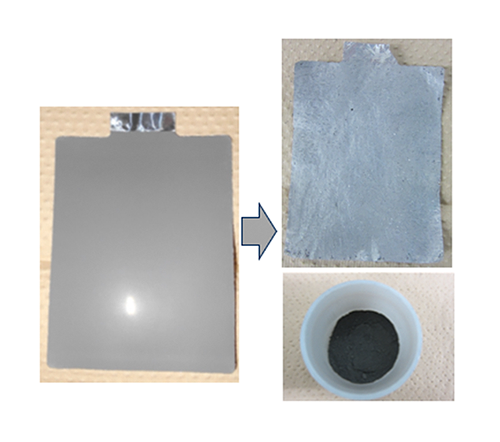
Maintaining the structural stability of the active material during the recycling process is important to achieve direct recycling. Among oxide anode materials, niobium titanium oxide (NTO) anode developed by Toshiba has a stable active material structure. Taking advantage of this characteristic, Toshiba has developed a method whereby heat treatment decomposes the binder component that fastens the NTO within the anode, enabling stripping from the current collecting foil and easy separation and recovery (Figure 1).
Furthermore, this method enables processing at lower temperatures compared with the heat treatments typically used in electrode recycling, and the recovered active material can be reused directly after removing impurities.
Toshiba has manufactured electrodes using NTO recycled from simulated electrode waste produced during battery manufacturing processes as well as from batteries with simulated degradation up to their end of life. After evaluating their performance in batteries, it was confirmed that the active material capacity, an indicator of active material performance, maintains over 97% performance, comparable to a new battery.
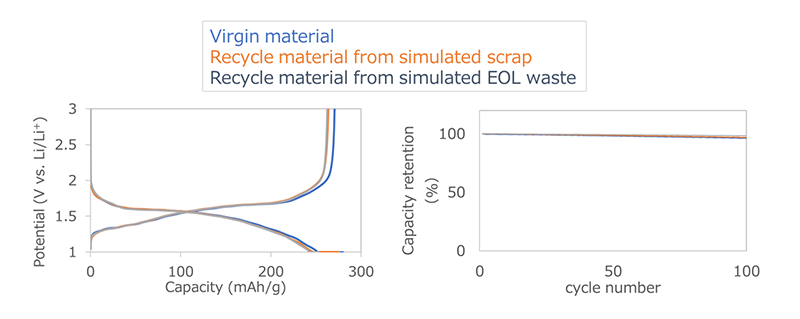
Active material capacity (left), cycle characteristics (right)